molar mass sodium chloride|how to calculate molar mass : Pilipinas There are 4 easy steps to find the molar mass of NaCl based on its chemical formula. 1. Count The Number of Each Atom. The first step to finding the molar mass of Sodium Chloride is to count the number of each atom present in a single molecule using the . WEB17 de mai. de 2023 · Cómo jugar a Lucky Jet Las reglas básicas Cómo funcionan las rondas. Lucky Jet es bastante sencillo de entender. Cada ronda ofrece diferentes .
0 · what is the molar mass of nacl
1 · sodium chloride melting point
2 · sodium chloride formula weight
3 · sodium chloride boiling point
4 · molecular weight of sodium chloride
5 · mass of nacl obtained
6 · how to calculate molar mass
7 · formula mass of sodium chloride
8 · More
webColourful and fun Karamba is the latest casino to open its doors to Canadian players. Discover what it has to offer in terms of games, bonuses, rewards and more, by checking .
molar mass sodium chloride*******There are 4 easy steps to find the molar mass of NaCl based on its chemical formula. 1. Count The Number of Each Atom. The first step to finding the molar mass of Sodium Chloride is to count the number of each atom present in a single molecule using the .
In solid sodium chloride, each ion is surrounded by six ions of the opposite charge as expected on electrostatic grounds. The surrounding ions are located at the vertices of a regular octahedron. In the language of close-packing, the larger chloride ions (167 pm in size ) are arranged in a cubic array whereas the smaller sodium ions (116 pm ) fill all the cubic gaps (octahedral voids) betwee.One mole of any compound will have a mass that is numerically equal to its molecular mass or formula mass and expressed in units of grams. This mass is also called the .
NaCl molecular weight. Molar mass of NaCl = 58.44277 g/mol. This compound is also known as Sodium Chloride. Convert grams NaCl to moles. or. moles NaCl to grams. .Formula: ClNa. Molecular weight: 58.443. IUPAC Standard InChI: InChI=1S/ClH.Na/h1H;/q;+1/p-1. IUPAC Standard InChIKey: FAPWRFPIFSIZLT . Enter the chemical formula of a compound and get its molar mass in g/mol. Learn how to calculate molar mass using the general formula and examples of NaCl, . The molar mass of a substance is the mass in grams of 1 mole of the substance. As shown in this video, we can obtain a substance's molar mass by .Need to know the atomic mass of a sodium chloride molecule? Our molar mass calculator uses the periodic table and the chemical formula to solve for the molar mass .1. 60.6628. Computing molar mass step by step. First, compute the number of each atom in NaCl: Na: 1, Cl: 1. Then, lookup atomic weights for each element in periodic table: Na: 22.98976928, Cl: 35.453. Now, compute the sum of products of number of atoms to the atomic weight: Molar mass (NaCl) = ∑ Count i * Weight i =.Sodium Chloride molecular weight. Molar mass of NaCl = 58.44277 g/mol. Convert grams Sodium Chloride to moles. or. moles Sodium Chloride to grams. Molecular weight calculation: 22.989770 + 35.453. Percent composition by element. Element: Sodium Symbol: Na Atomic Mass: 22.989770 # of Atoms: 1 Mass Percent: 39.337%.The first step to finding the molar mass of Sodium Chloride is to count the number of each atom present in a single molecule using the chemical formula, (Na)Cl: Element Number of Atoms; Na (Sodium) 1: Cl (Chlorine) 1: 2. Find Atomic Mass of Each Element.
The first step to finding the molar mass of Sodium Chloride is to count the number of each atom present in a single molecule using the chemical formula, Cl(Na): Element Number of Atoms; Cl (Chlorine) 1: Na (Sodium) 1: 2. Find Atomic Mass of Each Element.
molar mass sodium chlorideNotes. Go To: Top Data from NIST Standard Reference Database 69: NIST Chemistry Book The National Institute of Standards and Technology (NIST) uses its best efforts to deliver a high quality copy of the Database and to verify that the data contained therein have been selected on the basis of sound scientific judgment.how to calculate molar mass David Park. 4 years ago. First, you can calculate the molar mass of FeCl2 by adding the molar masses of Fe (55.845 g/mol) and 2 atoms of Cl (2 times (35.446 g/mol). This gives a molar mass of 126.737 g/mol. Since each mole is 126.737 grams, you multiply 3.5 mols by 126.737 grams, giving you 443.58 grams.
The molar mass will be equal to: (1 atom x 56 grams/mole Fe) + (2 atoms x 35.5 grams/mole of chlorine) = 127 grams/mole of iron (II) chloride. For other compounds, this might get a little bit more complicated. For example, take the example of zinc nitrate, or Zn (NO 3) 2. In this compound, we have one atom of zinc, two atoms of nitrogen (one .
Find the molar masses of carbon (C), hydrogen (H), and oxygen (O). Count the number of atoms of each element in the compound. Find the molar mass of glucose by multiplying the atomic masses of the atoms and their number, then find the sum: μ = 6 × 12.01 g/mol + 12 × 1.0079 g/mol + 6 × 16 g/mol = 180.1548 g/mol.Sodium Chloride molecular weight. Molar mass of NaCl = 58.44277 g/mol. Convert grams Sodium Chloride to moles. or. moles Sodium Chloride to grams. Molecular weight calculation: 22.989770 + 35.453. Percent composition by element. Element: Sodium Symbol: Na Atomic Mass: 22.989770 # of Atoms: 1 Mass Percent: 39.337%.Step1. Molar mass. The mass of 1 mole of a substance is called its molar mass. Step2. Formula for molar mass. Molar mass = The atomic mass of element × number of atoms given in subscript. Step3. Molar mass of NaCl. Molar mass of NaCl = Atomic mass of Na+ Atomic mass of Cl = 22. 99 + 35. 45 g mol-1 = 58.44 g mol-1. Hence, the molar mass of .As an example, consider sodium chloride, NaCl, the chemical name for common table salt. Sodium chloride is an ionic compound composed of sodium cations, Na +, and chloride anions, Cl −, combined in a 1:1 ratio. The formula mass for this compound is computed as 58.44 amu (Figure \(\PageIndex{3}\)).Molar concentration, also known as molarity, and can be denoted by the unit M, molar. To prepare 1 L of 0.5 M sodium chloride solution, then, as per the formula, use 29.22 g of sodium chloride (0.5 mol/L * 1L * 58.44 g/mol = 29.22 g). The mass molarity calculator tool calculates the mass of compound required to achieve a specific molar .The NaCl Molecular Weight (Sodium Chloride) is 58.44 g/mol. Visit BYJU'S to understand the properties, structure, and uses of Sodium Chloride (NaCl) explained by India's best teachers. . Sodium chloride: Molecular Weight/ Molar Mass of sodium chloride: 58.44 g/mol: Density of sodium chloride: 2.165 g/cm 3: Boiling Point of sodium chloride: 1. . Explanation of how to find the molar mass of NaCl: Sodium chloride.A few things to consider when finding the molar mass for NaCl:- make sure you have the cor.As an example, consider sodium chloride, NaCl, the chemical name for common table salt. Sodium chloride is an ionic compound composed of sodium cations, Na +, and chloride anions, Cl −, combined in a 1:1 ratio. The formula mass for this compound is computed as 58.44 amu (Figure \(\PageIndex{3}\)).
Molar concentration, also known as molarity, and can be denoted by the unit M, molar. To prepare 1 L of 0.5 M sodium chloride solution, then, as per the formula, use 29.22 g of sodium chloride (0.5 mol/L * 1L * 58.44 g/mol = 29.22 g). The mass molarity calculator tool calculates the mass of compound required to achieve a specific molar .The NaCl Molecular Weight (Sodium Chloride) is 58.44 g/mol. Visit BYJU'S to understand the properties, structure, and uses of Sodium Chloride (NaCl) explained by India's best teachers. . Sodium chloride: Molecular Weight/ Molar Mass of sodium chloride: 58.44 g/mol: Density of sodium chloride: 2.165 g/cm 3: Boiling Point of sodium chloride: 1. . Explanation of how to find the molar mass of NaCl: Sodium chloride.A few things to consider when finding the molar mass for NaCl:- make sure you have the cor.
The molar mass of sodium chloride (NaCl) is determined by adding the molar masses of sodium (Na) and chlorine (Cl). The molar mass of Na is 22.99 amu and that of Cl is 35.45 amu. So, the molar mass of NaCl = 22.99 amu (Na) + 35.45 amu (Cl) = 58.44 amu.
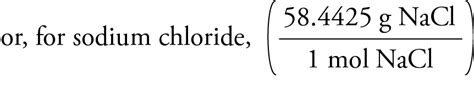
You need to find the molar mass of Na and Cl. If you look straight into the periodic table, you can identify it immediately. Na = #23.0 g mol^-1# Cl = #35.5 g mol^-1# Step 3: Add the component parts up and it will give you the molar mass of the compound (NaCl). 23.0 + 35.5 = 58.5. Therefore, the molar mass of Sodium Chloride (NaCl) is .
If we had a saturated solution of sodium chloride at 25 ˚C, we could quote the concentration as 359 grams/L, but because we know the molar mass of sodium chloride (58.44 grams/mole), we could also express our concentration as: ⎛⎝(359g) × 1mole 58.44g 1L ⎞⎠ = 6.14moles/L ( ( 359 g) × 1 m o l e 58.44 g 1 L) = 6.14 m o l e s / .
There is one Sodium atom and one Chlorine atom in the formula for Sodium Chloride(NaCl). As a result, the molar mass of sodium chloride will be equal to the sum of the two atoms’ molar masses. Sodium has an atomic mass of 22.99 g/mol, while chlorine has an atomic mass of 35.45 g/mol. As a result, sodium chloride’s molar .
As an example, consider sodium chloride, NaCl, the chemical name for common table salt. Sodium chloride is an ionic compound composed of sodium cations, Na +, and chloride anions, Cl −, combined in a 1:1 ratio. The formula mass for this compound is computed as 58.44 amu (see Figure 3.4).
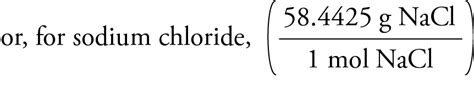
Molar mass: 58,44277 g/mol. The number of atoms: 2 - Na, Cl. Melting point: 800.8 ° C. Boiling point: 1465 ° C. Solubility: in water, in methanol, in ammonia. Sodium chloride is the sodium salt of hydrochloric acid. It is included in table salt as the main component. It is present in large quantities in seawater and is a precondition for its .3.24. Table salt (sodium chloride) (NaCl) 1. 0.82. 0.23. 0.17. Use this easy tool to quickly convert Table salt (sodium chloride) as a unit of Molar mass.We can use the rearranged molarity equation to calculate the moles of NaCl needed for the specified concentration and volume: mol NaCl = [ NaCl] × L of solution = 0.800 mol L × 0.250 L = 0.200 mol NaCl. We can then use the molecular weight of sodium chloride, 58.44 g mol , to convert from moles to grams of NaCl :
Resultado da Amirdrassil Raid Bosses and Loot iLvl on Heroic Difficulty. Defeating .
molar mass sodium chloride|how to calculate molar mass